Understanding basics of soil pH
When you visit garden centers, nurseries or while talking to garden professionals you often hear about testing soil pH, but what it really means?
What is pH?: In simple terms, pH is about measuring how acid or alkaline a substance is? pH is measured on a logarithmic scale of 0 to 14 with a value of 7 being neutral, less than 7 is acidic and more than 7 becomes alkaline.

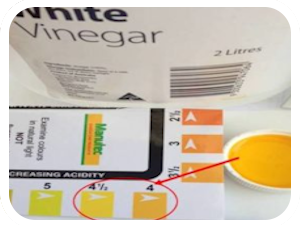
We use a number of acidic and alkaline (caustic) and neutral household materials in our daily life. For example, Vinegar or Lime juice are very acidic (pH 4 or less) where as the bicarb soda or washing powders (detergents) are very alkaline (pH more than 8.5). The pH of pure water will normally be around neutral range.


Most plants grow best in soils that are slightly acidic to neutral range of pH 6 to 7.
However there are few exceptions like acid loving plants (such as Azalea, Camellias, Blue berries etc.) can grow under acidic conditions with pH 5 to 6, but not many garden plants prefer alkaline conditions although a few plants can tolerate slightly alkaline condition.
What is Soil pH?
Soil pH is about measuring how acid or alkaline the soil condition is?
Soil pH is very crucial as it directly influences the root activity and absorption of nutrients which are both important in determining plant’s growth. When we touch strong acid or caustic soda we experience burning or irritation, similarly plant roots are also very sensitive and are affected by excessive acidity (low pH) and alkalinity (high pH).
Below is an illustration of 3 kinds of soils tested using Manutec Soil pH Test Kit

